a face lift for the soft palate™

An estimated 1 in 4 adults suffer from a clinically harmful level of snoring.
Snoring is caused by an airway obstruction.
In many cases, the source of the airway obstruction is at the top and back of the mouth, (soft palate tissue), which relaxes and falls toward the tongue during sleep.
Zelegent has developed a technology that allows physicians to quickly and safely alleviate some cases of snoring through an office-based treatment.
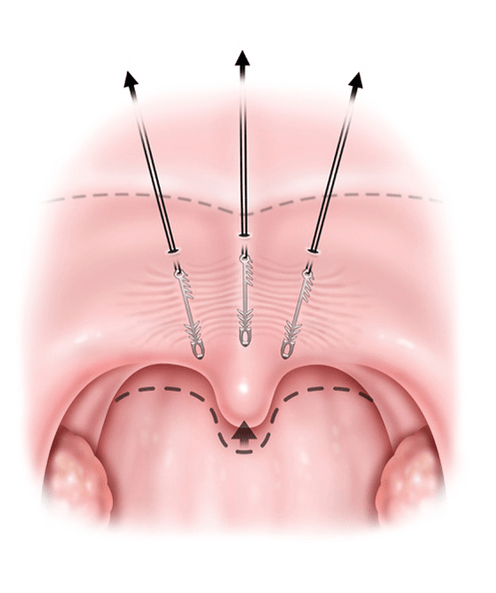
Simply put, the procedure works like "a face lift for the soft palate."™
introducing Elevoplasty®
A minimally-invasive procedure performed in a doctor's office that lifts soft palate tissue to
reduce or eliminate snoring.*
Minimally
Invasive
Procedure
The minimally-invasive procedure is performed in-office under local anesthetic and involves no downtime.
Completed in
Approximately
10 Minutes
Treatment for a vexing healthcare challenge can be completed in an approximate ten-minute office procedure.
An
Anatomic
Remedy
The soft palate is lifted to anatomically fix a root cause of snoring without the need to wear a treatment device during sleep.
latest news


Zelegent Acquires MISH Technology Platform, Extending Product Pipeline into Obstructive Sleep Apnea Therapeutics